Abstract
Background: Elderly patients (pts) diagnosed with classical Hodgkin lymphoma have poorer outcomes relative to younger counterparts. Elderly pts have comorbidities which contributes to treatment delays and higher rates of toxicities, leading to poor clinical outcome. Frailty measurements are subjective and cumbersome to perform in busy clinical settings. Body composition analysis based on CT has shown prognostic value in several malignancies in this age group. We therefore report the findings of body composition analysis in elderly cHL pts in the frontline setting.
Methods: Pts aged ≥60 with newly diagnosed cHL treated with combinations of anti-PD1 antibody nivolumab (Nivo), brentuximab vedotin (Bv), and/or chemotherapy between 2014 and 2021 were queried. We excluded pts with concomitant malignancies or unavailable baseline imaging. Patient characteristics were scored. Baseline axial CT images at the level of 3rd lumbar vertebra (L3) were segmented using MIM 7.2 (MIM Software Inc, Cleveland, OH). Cross sectional areas of skeletal muscle (SKM), subcutaneous adipose tissue (SAT), and visceral adipose tissue (VAT) were measured based on densities of −29 to +150, −190 to −30, and -150 to -50 hounds field units (HUs), respectively. Total adipose tissue (TAT) was obtained as sum of VAT and SAT. An index of each was calculated by normalization for stature (height in m2). Radiation attenuation of skeletal muscles density (SMD) was measured as mean HUs at the same level. The primary end point was progression free survival (PFS). Receiver operating characteristics (ROC) curves were used to determine optimal cutoff points. Kaplan-Meier analyses were used to compare survival. P<0.05 was considered statistically significant. EOT response assessment was done according to Lugano 2014 criteria.
Results: A total of 63 newly diagnosed elderly cHL patients were screened and 33 patients met the eligibility criteria, males were 20 (61.5%), median age 73.8, range (71.2, 77.2), advanced stage 24 (72%). Fifteen (45.4%) were treated with AVD/ABVD, 12 (36.4%) Bv-AVD/AD and 6 (18.2) Nivo Bv. Response at interim PET (iPET) was negative in 26 (78.8%). Complete remission at EOT was observed in 28 (84.8%) patients.
Among body composition variables, SMD showed consistent correlation with survival in both males and females (AUC=0.76 and 0.76, respectively). Cutoffs of 40 HU in males and 28 HU in females were used to group the patients into high- and low-SMD. The SMD was not significantly different between treatment groups (P=0.64) and distribution of age and BMI were the same in low and high SMD (P= 0.84, and 0.81, respectively). Patients with low SMD had a CR rate of 69.2% compared to 95% in the high SMD patients (P<0.05), while patients with negative iPET achieved 91% CR at EOT compared to 71.4% in positive iPET (P=0.20).
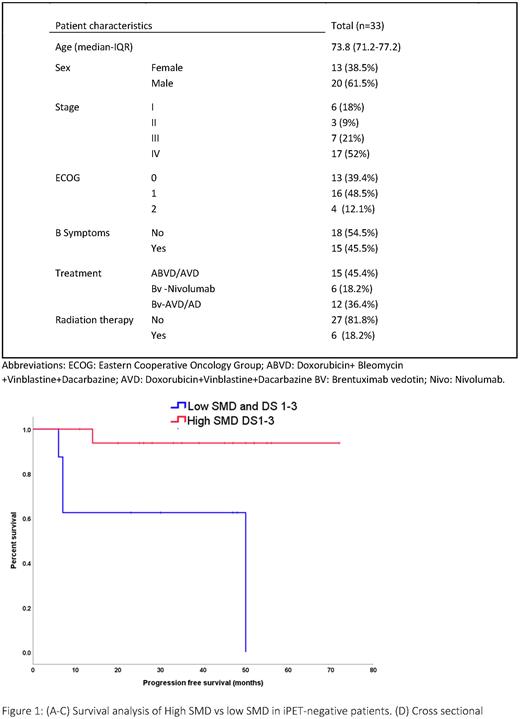
The mean follow-up time of the study cohort was 55.7 months (CI 95% 46.2, 65.4). High SMD group had a 48-months PFS of 89% vs 58% in pts with low SMD (P=0.013). Combining SMD and iPET status allowed for enhanced stratification of the iPET-negative group: pts with high SMD and iPET negative iPET had 93% PFS compared to 62.5% PFS in low SMD and iPET negative iPET at 48 months (P<0.01) (Figure-1)
Conclusion: Our results suggest that SMD may add value to the prognostication in elderly pts with cHL. In addition, the incorporation of SMD with iPET could identify a cluster of pts with low SMD who had worse prognosis compared to those with high SMD. This may allow for better treatment adjustments in this population.
Disclosures
Ahmed:Merck: Research Funding; Seagen: Research Funding; Tessa Therapeutics: Consultancy, Research Funding; Xencor: Research Funding; Chimagen: Consultancy, Research Funding; Servier: Membership on an entity's Board of Directors or advisory committees; Myeloid Therapeutics: Consultancy. Nair:Incyte Corporation: Honoraria. Steiner:BMS: Research Funding; Seagen: Research Funding; GSK: Research Funding; Rafael Pharmaceuticals: Research Funding. Wang:AbbVie: Consultancy; Acerta Pharma: Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; BioInvent: Consultancy, Honoraria, Research Funding; Deciphera: Consultancy; InnoCare: Consultancy, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Kite Pharma: Consultancy, Honoraria, Research Funding; Leukemia & Lymphoma Society: Consultancy, Honoraria; Lilly: Consultancy, Research Funding; Merck: Honoraria; Milken Institute: Consultancy; Oncternal: Consultancy, Research Funding; Pepromene Bio: Consultancy; Pharmacyclics: Consultancy, Honoraria, Research Funding; VelosBio: Consultancy, Research Funding; Celgene: Research Funding; Genmab: Research Funding; Genentech: Consultancy, Research Funding; Juno Therapeutics: Consultancy, Research Funding; Loxo Oncology: Research Funding; Molecular Templates: Research Funding; Vinverx: Research Funding; Dava Oncology: Honoraria; Eastern Virginia Medical School: Honoraria; IDEOlogy Health: Honoraria; LLC TS Oncology: Honoraria; Medscape: Honoraria; Meeting Minds Experts: Honoraria; MJH Life Sciences: Honoraria; Moffit Cancer Center: Honoraria; OncLive: Honoraria; Physicians Education Resources (PER): Honoraria; Practice Point Communications (PPC): Honoraria; Studio ER Congressi: Honoraria; Oncology Specialty Group: Honoraria. Pinnix:Merck Inc: Research Funding. Lee:Cancer Experts: Honoraria; Korean Society of Cardiology: Honoraria; Curio Science: Honoraria; Olson Research: Honoraria; Aptitude Health: Honoraria; Deloitte: Honoraria; Guidepoint Global: Honoraria; Janssen: Honoraria; Briston-Myers Squibb: Research Funding; Celgene: Research Funding; Octernal Therapeutics: Research Funding; Seagen: Research Funding; Takeda: Research Funding; Pharmcyclics: Research Funding; Century Therapeutics: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal